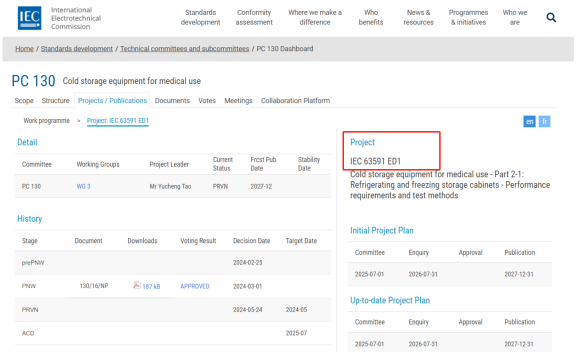
Recently, the IEC international standard proposal "Cold Storage Equipment for Medical Use - Part 2-1: Refrigerating and freezing storage cabinets - Performance requirements and test methods," jointly submitted by Haier Biomedical and CVC Wikai, has been successfully approved.
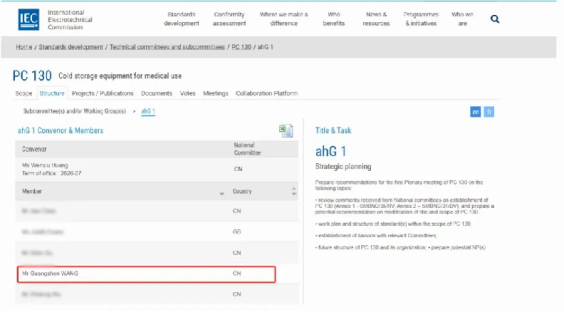
Previously, leveraging its global resources, Haier Biomedical assisted in establishing the International Electrotechnical Commission's Project Committee on Medical Cold Storage Equipment (IEC/PC 130).
Last November, Guangsheng Wang, Deputy General Manager of Haier Biomedical, participated in the PC130 launch meeting as the only expert from a manufacturing enterprise in China within the ahG1 Strategic Planning Working Group.

At the meeting, Haier Biomedical actively contributed insights to the future work and standard system construction of PC130. Experts from various countries agreed to initiate two international standard projects: terminology classification, and cold storage equipment for medical use, with China proposing the project establishment.
In recent years, medical cold storage equipment has become crucial for ensuring the safe transportation and storage of vaccines, medicines, and other biological samples. However, there has been no specialized technical institution or universal standard for medical cold storage equipment, leading to inconsistency in product standards in international trade.
The approved proposal "Cold Storage Equipment for Medical Use - Part 2-1: Refrigerating and Freezing Storage Cabinets - Performance Requirements and Test Methods" specifies technical indicators and corresponding test methods for medical refrigerating and freezing storage cabinets. The technical indicators include temperature setting deviation, temperature uniformity, temperature fluctuation, cooling rate, power failure temperature rise time, and abnormal alarm. The approved proposal provides a unified standard for the design, development, production, and application of these storage cabinets.
The establishment of this international standard will not only provide a basis for quality management in the storage and transportation of medical products, but also become a positive demonstration and safeguard in new technology development. It will support the orderly conduct of global trade while enhancing the international competitiveness of China's medical cold storage industry.
Currently, Haier Biomedical's products and solutions are applied in over 150 countries and regions worldwide. Haier Biomedical will continue to drive technological innovation and the development of international standards, contributing to healthcare, making life better through the intelligent protection of life science.












.png)