World’s First Vaccine
Edward Jenner was born in Berkeley, England about three centuries ago amidst a grave health crisis, during which a highly contagious and fatal disease known as “smallpox” plagued the populace, killing nearly 45,000 people annually in the United Kingdom alone. Upon returning to his hometown, Jenner, who had dedicated himself to the study of anatomy and medicine from 12 years old, discovered cowpox, a disease exhibiting symptoms of striking resemblances to smallpox. After devoting nearly 20 years of his life to thorough observation and meticulous analysis, he daringly proposed that “inoculating humans with cowpox pus could potentially give them the ability to fight smallpox.”

In 1796, Jenner used a clean knife to create multiple minor incisions on the arm of a young boy and administered drops of cowpox pus onto these wounds, thereby pioneering what is now recognized as the first vaccine for humans. The boy had successfully recovered from the mild symptoms of cowpox, and avoided any subsequent infection with the smallpox virus. Through his research, Jenner had successfully demonstrated the correlation between cowpox and immunity against smallpox, and as a result, he was honored as the Father of Immunology.
Cold Chain Transportation of Vaccines
Over the course of the past two centuries since the discovery of vaccines, significant technological advancements have fueled the development of a wide array of vaccine types. Consequently, the vaccine transportation industry has emerged to cater to the rising demand for vaccines. From the initial production stages to the final inoculation process, the vaccine cold-chain transportation sector presents huge business prospects. Available statistics show that the successful development of the COVID-19 vaccine has resulted in a significant surge in the cold chain transportation market; the growth rate this market experienced surpassed 7.2 times its previous value, and the potential market space for this industry is in the range of tens of billions of dollars. According to industry insiders, the launch of the COVID-19 vaccine will usher in a favorable period of transformation and advancement for the entire pharmaceutical cold chain industry.
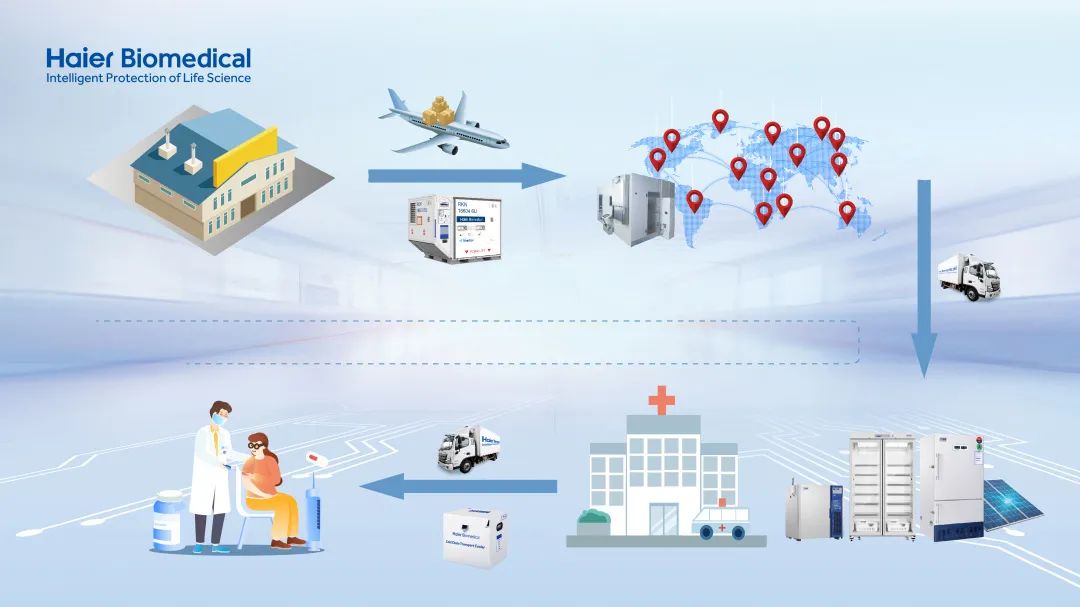
However, the effectiveness of vaccines can be compromised by various factors, including high temperature, freezing conditions, and prolonged exposure to light. Therefore, it is imperative that they are stored, transported, and used within specific temperature conditions to maintain the integrity of vaccines throughout the transportation process. In other words, an uninterrupted cold chain must be ensured from the manufacturers to the vaccination units, with real-time monitoring. To achieve this, it is essential to formulate a transportation plan in advance.
Storage of Vaccines
To maintain the activity of the vaccines, it is crucial to store them in a controlled temperature environment, as they are biological products that require specific temperature conditions. Strict adherence to storage standards for different vaccines is also crucial, both within laboratory settings and vaccination units. The storage conditions for vaccines vary depending on their respective characteristics and manufacturing processes. Inactivated vaccines, viral vector vaccines, and recombinant protein vaccines are typically stored under temperatures between 2°C and 8°C, while live attenuated vaccines are stored under temperatures typically below -20°C. Meanwhile, mRNA vaccines, which are the third generation of vaccines following inactivated, live attenuated, and viral vector vaccines, exhibit comparatively lower stability compared to other vaccine types. For example, Pfizer’s vaccine requires storage at ultra-low temperatures, ranging from -80°C to -60°C, and remains stable for only 2 hours after thawing at room temperature, and Moderna’s Spikevax vaccine, while relatively more stable, still requires storage within a -20°C environment.

The World Health Organization predicts that on a global scale, about 50% of vaccines are wasted every year, which is largely due to the inadequate availability of temperature control systems and equipment facilities. The pharmaceutical cold chain transportation sector holds a prominent position within the cold chain industry market, and in particular, the vaccine transportation within the cold chain has high and stringent requirements to maintain optimal conditions throughout the entire process. According to Air Cargo International, “Temperature will be one of the biggest challenges in transportation.”
How To Ensure Vaccine Safety?
So, how can we ensure the safety of vaccines during transportation and storage? To address this concern, Haier Biomedical has offered a comprehensive range of vaccine cold chain solutions, which not only encompass products that cater to all temperature requirements, but also include advanced vaccine stockpile monitoring solutions, allowing for 24-hour temperature monitoring and automatic alarms for any irregularities, thereby maximizing vaccine safety.
First, transportation and storage products should be equipped with cutting-edge international advanced RFID technology, which is a wireless communication technology that enables the identification of targets through non-contact data communication between the reader and the tag. Through this technology, users can not only monitor the temperature of vaccines in real time, but also obtain accurate vaccine inventory information. It also optimizes the procurement process, effectively addressing the issue of stock build-up and vaccine shortages. For example, the Haier Biomedical HYC-390R RFID refrigerator, equipped with full process automation and intelligent control features, can automatically detect and monitor the information and access status of vaccines in real time, ensuring that all vaccines administered to patients are systematically managed. At the same time, the intelligent process control system improves the efficiency and reliability of vaccine management, and enables the monitoring of expiration dates and facilitates automatic inventory counting, thereby reducing the need for manual labor and minimizing the risk of management errors.
In addition, low-temperature storage and IoT technology should be incorporated throughout the entire vaccine storage process.The equipment should be equipped with an IoT monitoring module and an intelligent IoT sample management system that enables it to perform real-time monitoring and alarm functions for both the temperature inside the box and the operation status of the equipment, thereby ensuring vaccine safety round the clock. In June of this year, a janitor employed at the Rensselaer Polytechnic Institute turned off an ultra-low-temperature refrigerator, an action prompted by a malfunctioning alarm system, which ultimately led to the irreparable loss of 25 years’ worth of research and a financial setback of up to USD 1 million. This incident further highlights the necessity of 24-hour monitoring and excellent heat preservation capabilities for refrigerators used to store biological samples. The Haier Biomedical Ultra-Low Temperature Refrigerator has an impressive heat preservation time of more than 60 hours after power failure, which equips the refrigerator to effectively handle various emergency situations.
Meanwhile, by incorporating a display module, an information storage and processing module, and a BIMS system on the equipment, the Haier Biomedical Ultra-low Temperature Refrigerator can accurately identify and record the location where vaccines are stored based on their respective labels, a feat made possible by using a scanning system. This allows for users’ one-click synchronization and seamless access to vaccines, thereby greatly reducing the time investment required for their work. In addition, RFID technology employed during vaccine storage can play a crucial role in accurately controlling the storage and usage of vaccines, thereby safeguarding vaccine counts and vaccine safety, and ensuring that patients receive properly managed vaccines.

For economically disadvantaged and energy-deficient regions, Haier Biomedical focuses on research and development in solar-powered products and innovates zero-carbon technology for the development of solar vaccine refrigerators. At the same time, to mitigate the impact of equipment operation interruptions or failures in regions with limited energy resources, the company offers a range of solutions, such as the RTMD series that enables remote monitoring and control of high and low temperatures, door opening and closing, automatic logging and detection, real-time alarms, and intelligent management, and the DATA LOGGER temperature logger pen that records equipment temperature in real-time and promptly alerts users to any abnormal temperature fluctuations to ensure vaccine safety in all aspects. The company has also introduced a customized service system, aiming to enhance its products and cater to the specific requirements of its clients, which is expected to improve the convenience and universality of its products within the local region.
Over the centuries, vaccines have transcended the boundaries of time and space, making remarkable contributions to safeguarding the lives and health of people all over the world. Haier Biomedical has also made breakthroughs, successfully overcoming one challenge after another while swiftly expanding its industrial deployment, consistently introducing innovative and forward-looking products, and continuously improving its vaccine cold chain solutions. These efforts have significantly contributed Haier Biomedical’s knowledge and expertise to the establishment of a global leading medical and healthcare ecosystem.












.png)